8th Advanced GMP Workshop 2023
Virtual October 3, 2023
Flyer
Agenda
Speakers
Presentation

Best Practices - Responding to FDA 483 Inspection Observations - Carmelo Rosa - Director Division of Drug Quality, CDER-OMQ FDA
Download PDF
Cross Contamination Control Strategy with Case Study and regulatory expectations - Rahul Songire - Vice President, Central Quality, Zydus Group
Download PDF
Harnessing ERP for enhanced compliance - Raghuram Janapareddy - Partner & Managing Director of India, Tenthpin
Download PDF
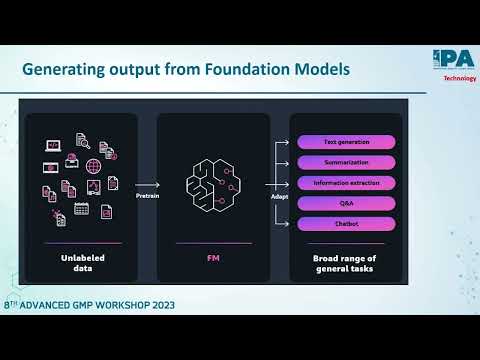
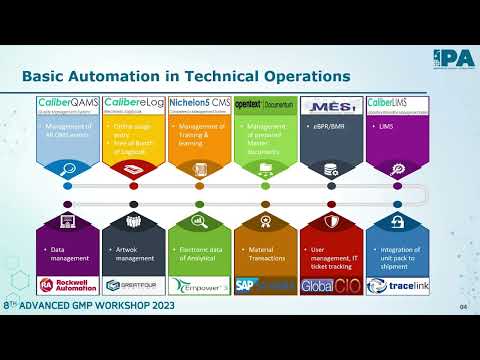
Role of Automation and Digitization in Manufacturing and Quality - Birendra Singh - President & Global Quality Head, Mankind
Download PDF
















 Watch Recording
Watch Recording